Veckan som snart tar slut har varit intensiv beträffande HoloMonitorhändelser.Bloggen har rapporterat om en upphandling från Taiwan, en ny studie om kombinationsläkemedel och ett samlat inlägg om just kombinationsläkemedelsstudier toppat med en sprillans ny filmsekvens som visar HoloMonitor in action vid läkemedelsframtagning.
Men dessa aktiviteter är inte alla.Undertecknad kan såhär på veckans sista dag rapportera om 3 händelser till.
Först har vi en ambitiös kinesisk studie (4/3 -2021) som handlar om cellers förmåga till omvandling/förvandling sett till utseende,form osv...Studien är ny information till världens cancerforskare.
Self-organization of Tissue Growth by Interfacial Mechanical Interactions in Multi-layered Systems
Abstract
Morphogenesis is a spatially and temporally regulated process involved in various physiological and pathological transformations. In addition to the associated biochemical factors, the physical regulation of morphogenesis has attracted increasing attention. However, the driving force of morphogenesis initiation remains elusive. Here, we show that during the growth of multi-layered tissues, morphogenetic process can be self-organized by the progression of compression gradient stemmed from the interfacial mechanical interactions between layers. In tissues with low fluidity, the compression gradient is progressively strengthened during differential growth between layers and induces stratification through triggering symmetric-to-asymmetric cell division reorientation at the critical tissue size. In tissues with higher fluidity, compression gradient is dynamic and induces 2D in-plane morphogenesis instead of 3D deformation accompanied with cell junction remodeling regulated cell rearrangement. Morphogenesis can be tuned by manipulating tissue fluidity, cell adhesion forces and mechanical properties to influence the progression of compression gradient during the development of cultured cell sheets and chicken embryos. Together, the progression of compression gradient regulated by interfacial mechanical interaction provides a conserved mechanism underlying morphogenesis initiation and size control during tissue growth.
Results (urval)
The behaviours of individual cells during cell sheet growth prior to the 3D morphogenesis transition were examined using a holographic imaging cytometer. Although the cell proliferation rate was negligibly altered during cell sheet expansion (Supplementary Figure S1a), the average area of individual cells significantly decreased, and the average cell thickness concurrently increased during cell sheet growth, indicating significant cell deformation (Figure 2f-h). Moreover, the largest cell deformation was observed at the central region of the cell sheet (Figure 2i-l). Since the cell sheet was grown from a single cell (monoclonal culture) and the chemical environment is uniform, the active cell contraction was presumably excluded and this cell deformation behaviour mainly reflected a passive a compression gradient within the cell sheet emerged during cell sheet growth. This phenomenon is consistent with previous reports29,30.
(a) The illustration of experimental workflow for studying the emergent 3D morphogenesis in a freely growing monolayer Hela cell sheet. (b) Representative phase contrast images of a growing monoclonal Hela cell sheet captured at the indicated time (d: day) after seeding. Scale bar: 100 μm. (c-d) XZ slice images of F-actin indicated by Lifeact-mCherry in a growing monoclonal Lifeact-mCherry+ Hela cell sheet captured at the indicated time after seeding. Scale bar: 50 μm. The statistical analysis of cell sheet thickness and diameter of a growing Hela cell sheet. (n = 10). (e) The critical size for 3D morphogenesis of growing cell sheet in different cell types. (f) The representative live images of a growing Hela cell sheet using HoloMonitor M4 time-lapse cytometer. Scale bar: 150 μm. (g) The statistical analysis of the area of individual cell during Hela cell sheet growth. (h) The statistical analysis of the thickness of individual cell during Hela cell sheet growth. (i) The magnified view of Hela cell sheet at 8 d in (b). Scale bar: 100 μm. (j) The statistical analysis of the individual cell area along the lines in (i). (k) Representative XY and XZ slice images of F-actin and nucleus stained by Phalloidin and DAPI respectively in Hela cell sheet. Scale bar: 20 μm. (l) The statistical analysis of the individual cell thickness along the lines in (i).
Cell division reorientation induces tissue stratification at critical compression condition
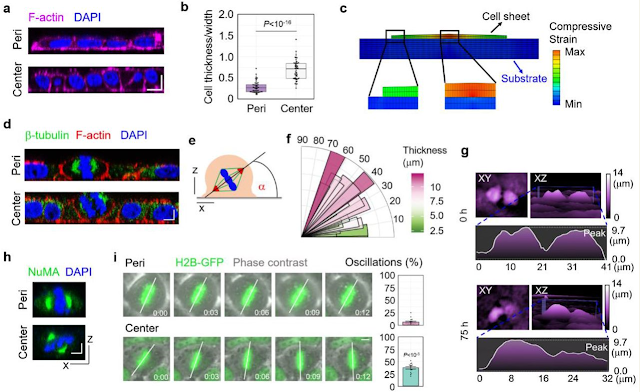
We proceeded to investigate the biological mechanism of the cell layer number increase in the central region of the cell sheet induced by cell layer/substrate interfacial interaction. We found that, in contrast to symmetric (parallel) cell division in the peripheral region of large cell colonies, abundant asymmetric (oblique or perpendicular) cell division was observed in the central cells which bore significant compressive strains (Figure 4a-f). Moreover, the orientation of the division plane was closely correlated with cell thickness (Figure 4f). This asymmetric cell division ultimately led at least one daughter cell to locate at the top layer of the cell sheet (Figure 4g and Supplementary Movies 1 and 2). The cell division reorientation was also confirmed by the subcellular localization of the nuclear-mitotic apparatus protein (NuMA), which is involved in the orchestration of mitotic spindle positioning 31 (Figure 4h). Moreover, spindle-rocking experiments indicate that cells in the central region showed a significantly higher level of metaphase plate oscillations which is always associated with asymmetric cell division 32 (Figure 4i). These results indicate that cell division reorientation from symmetric to asymmetric accompanied with compression gradient promotes tissue stratification.
Critical Compression triggers cell division reorientation to induce tissue stratification
(a) Representative XZ slice image of F-actin and nucleus stained by Phalloidin and DAPI respectively in the central region and peripheral region of Hela cell sheet. Scale bar: 10 μm. (b) The statistical analysis of cell deformation (thickness/width) in the center region and peripheral region of Hela cell sheet. (n = 50). (c) Cell shape variation induced by interfacial shear stress. (d) Representative XZ slice image of β-tubulin, F-actin and nucleus stained by β-tubulin antibody, Phalloidin and DAPI respectively in the central region and peripheral region of Hela cell sheet. Scale bar: 5 μm. (e) The schematic experimental setting of the mitotic spindle orientation. (f) Distribution of the spindle-axis angles of cells with different thickness in Hela cell sheet. (g) The representative images of dividing cells before (0 h) and after (75 h) critical compression during Hela cell sheet growth using HoloMonitor M4 time-lapse cytometer. (h) Representative XZ slice image of NuMA and nucleus stained by NuMA antibody and DAPI respectively in the central region and peripheral region of Hela cell sheet. Scale bar: 5 μm. (i) Analysis of spindle oscillation in in the central region and peripheral region of GFP-H2B+ U2OS cell sheet. The extent of oscillation was calculated and plotted in bar graphs on the right. Scale bar: 5 μm. (n = 14).
Materials and Methods
Live imaging
Live cell imaging was performed in Leica microscope or HoloMonitor M4, enclosed in an incubator to maintain the samples at 37 °C and 5% of CO2 throughout the experiments. Images were acquired every 10 min with Leica software. Spindle-rocking experiments were acquired every 3 min with Leica microscope. HoloMonitor M4 is a Quantitative phase imaging-based cell analyzer utilizing the principle of digital holographic microscopy. Live cell imaging was performed in HoloMonitor M4, enclosed in an incubator to maintain the samples at 37 °C and 5% of CO2 throughout the experiments. Images were acquired every 10 min with HStudio 2.7.
Image processing and Quantification
Supplementary Movies (tyvärr ej klickbara)
Supplementary Movie 1. Symmetric cell division before critical compression during Hela cell sheet growth visualized by HoloMonitor M4 time-lapse cytometer.
Supplementary Movie 2. Asymmetric cell division after critical compression during Hela cell sheet growth visualized by HoloMonitor M4 time-lapse cytometer.
Brazil Nut Effect Drives Pattern Formation in Early Mammalian Embryos
Abstract
The formation of three-dimensional ordered spatial patterns, which is essential for embryonic development, tissue regeneration, and cancer metastasis, is mainly guided by the chemical concentration gradient of morphogens. However, since no chemical concentration gradient has been observed in the early embryonic development (pre-implantation) of mammals, the pattern formation mechanism has been unsolved for a long time. During the second cell fate decision of mouse embryos, the inner cell mass (ICM) segregates into topographically regionalized epiblast (EPI) and primitive endoderm (PrE) layers. Here, we report that the segregation process of PrE/EPI precursors coincides with an emerged periodic expansion-contraction vibration of the blastocyst cavity, which induces phase transition in the ICM compartment to a higher fluidity state and generates directional tissue flows. By experiments and modeling, we demonstrate that the spatial segregation of PrE and EPI precursors is mediated by a "Brazil nut effect"-like viscous segregation mechanism in which PrE precursors with low affinity gradually migrate to the surface of ICM along with the tissue flow, while EPI precursors with high affinity remains inside ICM under cavity vibration. Artificially manipulation of the frequency and amplitude of cavity vibration could control the process of spatial separation as well as lineage specification of PrE/EPI. Furthermore, disruption of the cavity vibration in the initial stage after segregation could reverse the ICM cells back to a mixed state. Therefore, this study reveals a fundamental mechanism that guarantees the robustness of cell segregation and pattern formation without specific morphogens in early mammalian embryos. Our model also emphasizes a conserved function of cavity structure that widely exists in organisms as an energy reservoir and converter between different forms, such as chemical and mechanical energy.
Materials and Methods
3. Och slutligen kommer info från Ungern om en doktorsavhandling ledd av den HoloMonitorfrälste forskaren Robert Horvath.Doktoranden Kovács Kinga Dóra halvårsrapporterar om sin avhandling som till delar bygger på användande av HoloMonitor.Mina lingvistiska kunskaper i Ungerska är starkt begränsade men som jag förstår det är ämnet infärgningsfria cellstudier.
Bevezetés (Pdf som öppnas i nytt fönster)
Jelölésmentes bioszenzorok jelölőmolekula nélkül képesek a biológiai folyamatok nyomon
követésére. Az rezonáns rácsos hullámvezető alapú bioszenzorok kiválóan alkalmasak a felület
feletti rétegben érzékelni a törésmutató változást nagyon jó térbeli és időbeli felbontással. A
biomolekulák vagy élő sejtek felülethez történő adszorpciója során megváltozik a lokális
törésmutató, így ezek a folyamatok vizsgálhatók. Egyedi sejtek vizsgálata is lehetséges ezekkel
a bioszenzorokkal ennek a jelentősége abban rejlik, hogy eddig főként csak sejtpopulációkra
léteznek ilyen mérések. Az utóbbi évben kutatócsoportunk számos fontos lépéseket tett a fenti
módszerek kiterjesztésében nagyszámú egyedi sejtek párhuzamos vizsgálatára.
A kutatásom célja a biomolekulák szenzor-felülethez történő adszorpciójának és a
sejtadhézió folyamatának vizsgálata különböző biofizikai módszerekkel, mint FluidFM,
számítógépvezérelt mikropipetta, holomonitor és rezonáns rácsos hullámvezető alapú
bioszenzor, hangsúlyt fektetve a jelenségek egyedi sejt és populáció szintű vizsgálatára.
Min kommentar
Vi har alltså 6 Holo-aktiviteter för veckan.Även om det är tyst från Bolaget betyder det inte att det inte sker PHI-relaterat utanför sfären.Sen har vi en Q-rapport att se fram mot inom kort vilket kan förklara tystnad från deras kommunikationsavdelning.Men den nyhetstörstige vet vart man vänder sig om man vill bli uppdaterad av senaste nytt. 😎
Mvh the99

Inga kommentarer:
Skicka en kommentar