4 polska forskare + 1 jänkare har genomfört studier kring det endoplasmatiska nätverket, endoplasmanätet, eller endoplasmatiskt retikulum/retikel, förkortat ER (från latin reticulum endoplasmaticum "det lilla nätet inuti (cell)plasman", är en organell som kan uppta upp till 10 % av eukaryota cellers cytoplasma.Det endoplasmatiska nätverket bildar i en cell ett vätskefyllt hålrum, som är separerat från cytosolen (vätskan inuti cellen). Runt cellkärnan finns mycket endoplasmatiskt nätverk.
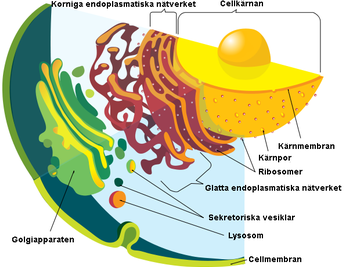 |
| Olika organeller i den eukaryota cellen. |
När ER blir utsatt för negativ påverkan (stress) påverkar det produktionen av proteiner som cellerna är beroende av för att fungera och vara livsdugliga. Forskarna har undersökt processen när detta händer och försökt hitta svar på lösning.
IRE1-mediated degradation of pre-miR-301a promotes apoptosis through upregulation of GADD45A
Abstract
The unfolded protein response is a survival signaling pathway that is induced during various types of ER stress. Here we focus on the IRE1 pathway to determine IRE1’s role in miRNA regulation during ER stress. During induction of ER stress in human bronchial epithelial cells, we utilized next generation sequencing to demonstrate that pre-miR-301a and pre-miR-106b, were significantly increased in the presence of an IRE1 inhibitor. Conversely, using nuclear-cytosolic fractionation on ER stressed cells, we found that these three pre-miRNAs were decreased in the nuclear fractions without the IRE1 inhibitor. We also found that miR-301a-3p targets the proapoptotic UPR factor, growth arrest and DNA-damage-inducible alpha (GADD45A). Inhibiting miR-301a-3p levels or blocking its predicted miRNA binding site in GADD45A’s 3’ UTR with a target protector increased GADD45A mRNA expression. An elevation of XBP1s expression had no effect on GADD45A mRNA expression. We also demonstrated that the introduction of a target protector for the miR-301a-3p binding site in GADD45A mRNA during ER stress promoted cell death in the airway epithelial cells. These results indicated that IRE1’s endonuclease activity is a two-edged sword that splices XBP1 mRNA for survival and degrades pre-miR-301a to elevate the mRNA expression of a pro-apoptotic gene, GADD45A.
Introduction
Endoplasmic reticulum (ER) homeostasis is dependent upon proper folding and maturation of secretory pathway proteins, lipid biosynthesis, and cellular calcium homeostasis. Disruption of the ER homeostasis, however, leads to a stress response called the unfolded protein response (UPR) that is a multifunctional signaling pathway (1–3). The UPR serves primarily as a cellular adaptive mechanism that counteracts the stress-related deregulation of ER function and promotes cellular survival from both intrinsic and extrinsic insults (4,5). If the recovery mechanisms are ineffective, however, and the ER stress is maintained, the UPR can promote cell death (6–10).
The UPR is mediated by three proximal ER stress sensors that include the inositol-requiring enzyme 1 alpha (IRE1), the PKR-like endoplasmic reticulum kinase (PERK), and the activating transcription factor 6 (ATF6) (6–10). Among them, IRE1 regulates the phylogenetically most conserved arm of the UPR that is involved in the balance between cell survival and cell death (11–13). IRE1’s unique feature is its UPR-related RNase activity that is responsible for the production of a potent transcription factor, X box-binding protein-1 (XBP1s). IRE1 regulates this activation through the nonconventional splicing of XBP1 mRNA and the degradation of a large pool of cytoplasmic mRNAs in a process called regulated-IRE1 dependent decay (RIDD) (9,14). The complex role of IRE1 in the regulation of mammalian UPR cannot be fully explained by IRE1’s one known, specific RNA target, X box-binding protein-1 (XBP1) or through the known mRNA substrates of its RIDD activity. Here, we focused on the role that IRE1 plays on miRNA expression during the UPR.
Using next-generation sequencing (NGS) of normal immortalized human bronchial epithelial cells, 16HBE14o-, we identified three miRNAs, hsa-miR-301a-3p, hsa-miR-106b-5p, and hsa-miR-17-5p that had decreased levels of expression during the UPR. Our analyses demonstrated that all 3 of their pre-miRNA precursors were degraded by IRE1. Furthermore, we demonstrated that the IRE1-mediated downregulation of hsa-miR-301a-3p allowed for the accumulation of a proapoptotic UPR factor, growth arrest and DNA-damage-inducible alpha (GADD45A), and this is known to contribute to cell fate decisions (15–17). The studies presented here demonstrate that during UPR, IRE1 degrades pre-miR-301a and this enhances the expression of GADD45A, and subsequently drives the ER stressed cells towards apoptosis. This study demonstrates how inhibition of miRNA maturation by IRE1 can modulate cell fate decisions during the UPR.
Materials and Methods (urval)
For real-time monitoring of cell viability, we applied real time and label free holographic microscopy-based monitoring of cell death and viability using HoloMonitor M4® time-lapse cytometer (Phase Holographic Imaging PHI AB, Lund Sweden). Holographic microscopy was used to follow the optical thickness and irregularity of cells exposed for up to 24 hours to Tm in the presence or absence of pre-miR-301a. The images from up to 5 independent optical fields were collected and analyzed according to manufacture instructions with HoloMonitor® App Suite software. Healthy cells are irregular in shape and thin, and dying cells are round and thick (30–35). For all analysis, the same cells parameters qualification was applied.
This data suggested that IRE1’s reduction of hsa-miR-301a-3p levels and the corresponding increases GADD45A expression could contribute to cell death decisions during ER stress. To test this, we performed real time and label free holographic microscopy-based monitoring of cell death and viability using a HoloMonitor® time-lapse cytometer. Holographic microscopy was used to follow the optical thickness and irregularity of cells exposed for up to 24 hours to Tm in the presence or absence of pre-miR-301a.(Figure 9)
 |
| Figure 9.IRE1-mediated degradation of pre-miR-301a during ER stress contributes to cell death decisions. |
The results of real-time monitoring of cell viability with the real time and label free holographic microscopy using a HoloMonitor M4® time-lapse cytometer of 16HEB14o- cells transfected with pre-miR-301a-3p or the scramble control and 48 hours later treated with Tm (2.5 µg/ml) up to 24 h. Images were collected every 15 minutes (from 5 independent optical fields), and the distribution of live (blue) and dying cells (red) as well as dead cells (grey) based on their optical thickness and irregularity is presented at the 12 and 24 hour time points. The images from up to 5 independent optical fields were collected and analyzed according to manufacture instructions with HoloMonitor® App Suite software. Representative samples are shown (A). For all analyses, the same cell parameters qualifications were applied. Experiments were performed in triplicate. Based on the cells irregularity and average optical thickness the percentages of healthy cells (B) and of dying cells (C) were calculated. Data represent the mean ± SD of three independent experiments. * P < 0.05 was considered significant. Schematic representation of role of IRE1-mediated degradation of pre-miR-301a on cell fate decision during ER stress (D).
 |
| Figure 10.hsa-miR-301a-3p modulates GADD45A levels during ER stress and contributes to cell death decisions. The results of real-time monitoring of cell viability using holographic microscopy (HoloMonitor M4® time-lapse cytometer). 16HBE14o- cells were transfected with target protector (Tp) or Tp control and treated with Tm (2.5 µg/ml) up to 24 h. Images were collected every hour from 5 independent optical fields. The distribution of live (blue), dying cells (red), and dead cells (grey) were based on their optical thickness and irregularity is presented only at the 6- and 12-hour time points (A). The images from up to 5 independent optical fields were collected and analyzed according to manufacture instructions with HoloMonitor® App Suite software. One representative optical field is presented in the right panels. For all of the analyses, the same cells parameter quantification was applied as in Figure 8. Experiments were performed in triplicates. Based on the cells irregularity and average optical thickness the percentages of healthy cells (B) and of dying cells (C) were calculated. Data represents the mean ± SD of three independent experiments. * P < 0.05 was considered significant. Schematic representation of role of IRE1-mediated degradation of pre-miR-301a on the cell fate decision during ER stress (D). |
Förklaringar och förslag på lösning hur återställa ER med dess funktion hittas i studiens avsnitt Discussions.
Här en studie som inte handlar om cancerforskning utan istället avancerad cellforskning där en påverkad ER har koppling till utvecklande av Alzheimers och Parkinsons sjukdom. Forskarna tror sig ha svaret på hur undvika det händelseförloppet. Excellenta HoloMonitor levererar igen.
Inga kommentarer:
Skicka en kommentar